The Mysteries of Effetre 069 Electric/Striking Yellow
![]()
I was in my studio one night working on taking pictures of some beads made with Glow-In-The-Dark Powders with a UV/Black light. Not being one to work on just one project at a time, also on the counter was a page from my Frit Reference project. It as open to a page where I had just glued in some beads fro a frit blend I had put together. I had the camera set up, the UV light on, everything laid out and the picture ready to take. I happened to glance over at the beads I had just glued into the reference book and one was fluorescing orange! What the heck? The frits I used were just some Effetre striking colors. Nothing in there should be fluorescing - especially Orange. To make matter even more interesting, not all of the tests for that blend were glowing - only the one that was Overheated. By overheated, I mean held in the flame longer than your normally would, but not necessarily taken to a higher temperature than normally would occur - just longer. The one that fluoresces is on E-204 white, but so are 3 of the others that don't. The colors in this blend are 069 Electric/Striking Yellow, 072 Striking Orange, and 076 Striking Red.
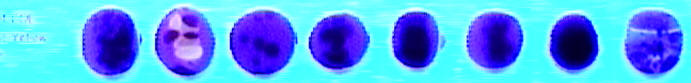
I checked the beads and it appeared that it was 069 Electric/Striking Yellow and so I checked the test beads for that color. Sure enough, some were fluorescing orange: Normal, Overcooked, and Reduced.
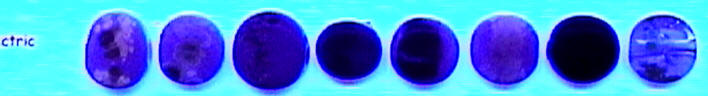
Here it is in a blend of 069 Electric/Striking Yellow and 456 Rubino. In this one the beads that are fluorescing are Normal, Overcooked, on Ivory, and Reduced.
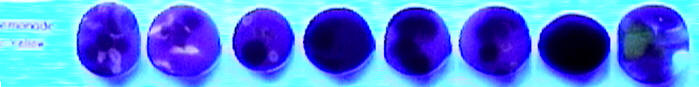
To help me figure out what's going on here, I put out a call for testers on Lampwork Etc. I asked them to make a bead and to use Effetre/Moretti 069 on it.
Here is what I know about this color:
The rods themselves do not fluoresce under a UV light. The Vetrofond version does, but I'm not really interested in them since they fluoresce in the first place. I'm interested in what goes on to change it from non-fluorescing to fluorescing.
It's a transparent color and when overheated, this glass becomes translucent. I thought that neither by itself fluoresces, but one bead came in that is a 069 Bead with a simple wrap of a same color stringer - and this one does fluoresce in spots. I later checked an old set of beads I made that included 2 simple spacer beads and one of them fluoresced and one didn't.
Uranium is not the chemical involved. The chemicals in this case are Silver Nitrate, Silver Carbonate or Silver Oxide to make it yellow and Germanium Dioxide to make it transparent. It works by diffusing micro-crystals of the silver throughout the glass matrix.
Dunham, Bandhu. Contemporary Lampworking. A Practical Guide to Shaping Glass in the Flame. Vol. 2. Prescott: Salusa Glassworks, 2003, 474.The effect is not dependent on the batch. I bought my striking yellow when I first started working with glass in June of 2001. Others who have submitted beads for this research bought theirs more recently - of course, I guess it could all be the same factory batch - if it's a slow mover.
I checked all the other Effetre colors and the Striking Red and Orange almost-kind-of-sort-of had a hint of a glow around the edges, but it wasn't enough to even tell if the fluorescence was orange or red. None of the other colors fluoresced at all.
|
The Beads That Didn't Fluoresce |
| Details |
|
|
|
|
|
|
|
|
|
|
|
![]()
What I now know about this glass
|
It doesn't seem to matter what torch is used. I have fluorescing beads made with a Hothead, Bobcat, Minor, and Mini-CC. |
|
It doesn't seem to matter what gas is used. I have fluorescing beads made with Bulk Propane, and Natural Gas. |
|
It doesn't seem to matter what oxygen source is used. I have fluorescing beads made with room Oxygen, tanked Oxygen, and with Oxygen from an Oxycons. |
|
It's not my "technique" that makes the difference since others can get the fluorscence as well. |
|
It doesn't NOT like copper. When used or mixed with anything or color containing copper, it turns black. |
![]()
It seemed to me that some colors would encourage the glass to fluoresce, and some wouldn't. In order to find out whether or not a color would, I decided to mix some up to see if the combination did. I made a bead of the resulting mix for each color in the Effetre/Moretti palate. Here are the resulting charts - the ones with the names highlighted in yellow were the mixes that fluoresced. Each mix is half and half. I did end up with enough interesting color mixes to make the charts interesting for the color mix project as well.
![]()
Some Technical Stuff
|
From Wikipedia:
|
|
From Encyclopædia Britannica:
|
|
From Electrical Engineering Training Series:
|
| The chemical formula for Germanium Oxide is GeO2 |
| According to Sylvania, the chemical formula they use in in lamps to fluoresce red is Mg4(F)GeO6:Mn |
|
From Yohkoh Analysis Guide: Not there anymore. the documents I was referencing is dynamic and keeps changing with every update.
|
![]()
I would like to thank the following people for submitting beads or contributing ideas:
![]()
You can contact Kitty by emailing her at kay@listen-up.org
© 2002-2023 - Kay R Powell. All rights reserved.